Hm Home
Porphyrins and Phthalocyanines
Materials Research Group
Au About Us
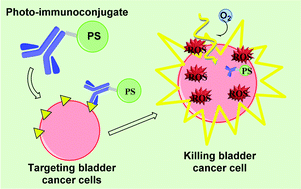
Glyco(porphyrins and phthalocyanines) for PDT
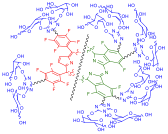 |
Synthesis, photophysical and photochemical features of novel water soluble galactodendrimeric-porphyrin and -phthalocyanine conjugates, incorporating a carbohydrate shell of eight and sixteen D-galactopyranose units, respectively. Chem. Commun., 2012, 48, 3608-3610.
|
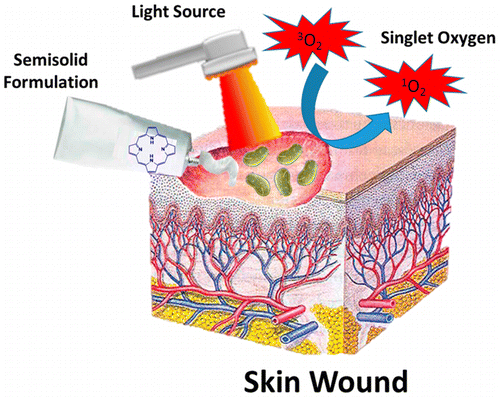 |
Semisolid formulations, such as gels, creams and ointments, have recently contributed to the progression of photodynamic therapy (PDT) and microbial photodynamic inactivation (PDI) in clinical applications. The most important challenges facing this field are the physicochemical properties of photosensitizers (PSs), optimal drug release profiles, and the photosensitivity of surrounding tissues. By further integration of nanotechnology with semisolid formulations, very promising pharmaceuticals have been generated against several dermatological diseases (PDT) and (antibiotic-resistant) pathogenic microorganisms (PDI). This review focuses on the different PSs and their associated semisolid formulations currently found in both the market and clinical trials that are used in PDT/PDI. Special emphasis is placed on the advantages that the semisolid formulations bring to drug delivery in PDI. Lastly, some potential considerations for improvement in this field are also discussed. |