|
Design and build multi-photonic components in a controlled intercomponent distance, orientation, and defined donor-acceptor ratios, with potential application as energy transfer and photonic materials. Design of multifunctional hybrid materials, such as porphyrin-phthalocyanine and porphyrin-fullerene C60 dyads for electronics, optics and/or light-harvesting applications.
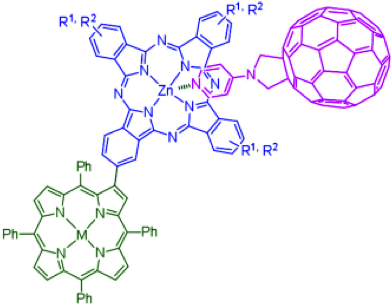 |
Photoinduced energy- and electron-transfer processes were studied in several porphyrin (P)/phthalocyanine (Pc)/fullerene (C60) hybrids in which the phthalocyanines were directly linked to the β-pyrrolic positions of the porphyrins and assembled through metal coordination with different pyridylfullerenes.
|
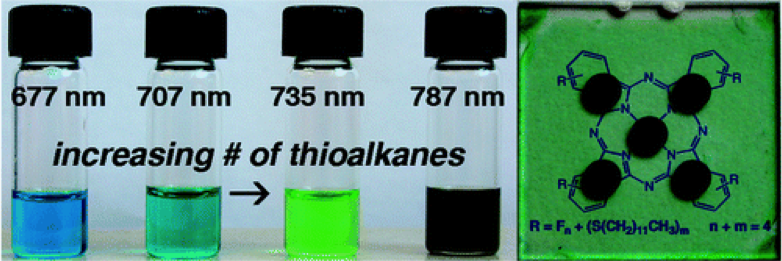
A core phthalocyanine platform allows engineering of the solubility properties the band gap, shifting the maximum absorption toward the red. A simple method for increasing the efficiency of heterojunction solar cells uses a self-organized blend of phthalocyanine chromophores fabricated by solution processing.
J. Am. Chem. Soc., 2010, 132, 2552–2554.
|
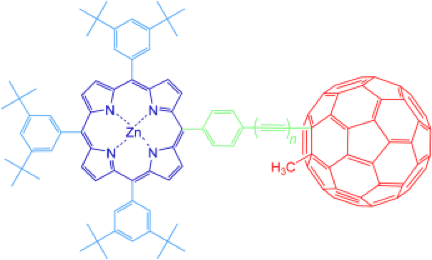 |
The synthesis and electrochemical and photophysical studies of a series of alkyne-linked zinc–porphyrin–[60]fullerene dyads are described. These dyads represent a new class of fully conjugated donor–acceptor systems. An alkynyl–fullerene synthon was synthesized by a nucleophilic addition reaction, and was then oxidatively coupled with a series of alkynyl tetra-aryl zinc–porphyrins with 1–3 alkyne units. Cyclic and differential pulse voltammetry studies confirmed that the porphyrin and fullerene are electronically coupled and that the degree of electronic interaction decreases with increasing length of the alkyne bridge.
Chem. Eur. J., 2005, 11, 3375–3388. |
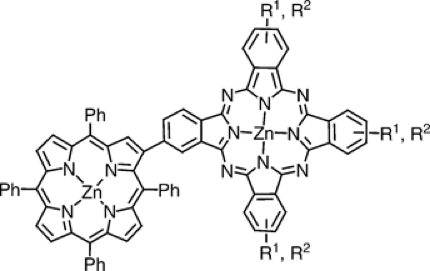
A series of novel porphyrin–phthalocyanine (Por-Pc) dyads have been synthesized by using standard methodologies for unsymmetrically substituted phthalocyanine preparation. These two chromophoric units have been directly linked for the first time, that is, without any spacer, through the β-pyrrolic position of a meso-tetraphenylporphyrin, thus allowing a close proximity of the two units in a rigid arrangement.
Eur. J. Org. Chem., 2006, 257–267. |
 |
|